A year of Kaizen at Sincrofarm: continuous improvement in the contract manufacturing of food supplements and medicines

Just over a year ago, at Sincrofarm we took an important step: we incorporated the Kaizen philosophy, one of the pillars of Lean Manufacturing, into our daily work in a structured way. Kaizen is a Japanese term that literally means: Kai (改): change Zen (善): good, for the better Not as a one-off project or […]
Looking back with gratitude: 2025 at Sincrofarm and the momentum towards 2026

2025 has been a year of growth, learning, and reaffirmation for Sincrofarm. We have made steady progress, not impulsively, but firmly, toward our goal of becoming a leading CDMO. We have not only met new challenges but also strengthened the foundations that sustain us: the trust of our team and our clients. In this review, […]
Arriving on time without compromising quality: the great challenge for CDMOs to close out the year
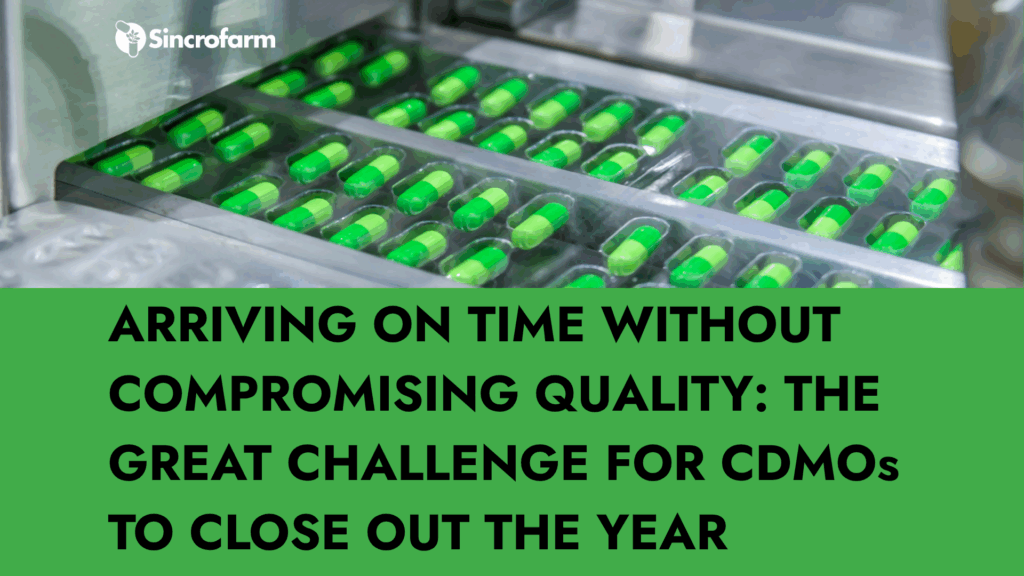
In the last quarter of the year, the pharmaceutical and nutraceutical industries face one of their most demanding periods. Companies accelerate product launches, prepare commercial campaigns, and strengthen their stock for the beginning of the following year. In this context, planning production in advance is not just advisable — it’s essential to ensure project success. […]
Why sincrofarm is more than a manufacturer

As we can see, the pharmaceutical and nutraceutical markets are constantly growing—and with that growth comes increased competition. This means that brands are becoming more demanding with their suppliers and partners, looking beyond just a service provider. They seek a technical, strategic, and committed ally—one who understands their challenges, adapts to their timelines, and helps […]
Quality certifications: the key to unlock international markets in food supplements

In the world of food supplements, it’s not enough to manufacture safe and compliant products—the real challenge lies in earning the trust of international markets, that’s where quality certifications play a decisive role. They are much more than just a stamp of approval—they’re the passport that enables brands to cross borders and compete in highly […]
Traceability in every manufacturing process: the foundation of safety and quality

In the manufacturing of pharmaceuticals and food supplements—under GMP and FSSC 22000 regulations respectively—traceability is much more than a legal requirement: it is the tool that guarantees every stage of the production process is controlled and documented in accordance with the certifications held by Sincrofarm. What is traceability, and why is it so important? Traceability […]
Why outsourcing the manufacturing of pharmaceuticals and food supplements can grow your brand?

In a highly regulated and increasingly competitive environment, choosing the right partner for the manufacturing of pharmaceuticals and food supplements is a key strategic decision. That partner must align with your values, quality standards, and business vision—because in this industry, quality is non-negotiable. Outsourcing manufacturing to a specialized CDMO (Contract Development and Manufacturing Organization) not […]
Vitafoods Europe 2025: See you at the industry’s key event

Who is Sincrofarm? We are a CDMO with over 35 years of experience in the manufacturing of pharmaceutical products and food supplements. We support our clients at every stage—from early development to final production—including regulatory affairs services, stability studies, material sourcing, and quality control. We operate under certified standards (GMP, FDA, FSSC 22000), with an […]
We are undertaking the digital transformation of Sincrofarm to improve operational efficiency and customer service.

Achieving greater operational efficiency, providing better customer service, and ensuring regulatory compliance are the goals we have set in the digital transformation process we are implementing at Sincrofarm. The steps we are taking in this journey are based on four main pillars: Tools: Under the premise “the core of the business at the center,” we […]
We are committed to implementing digital tools that bring agility and functionality to our processes

Digitalization as an added value to our main asset: people. With three decades in the industry, Sincrofarm is taking decisive steps towards digitalization, which provides functionality in our processes and enables us to streamline operations for the benefit of our customers. To achieve this, we have a team of experts in Information Technologies (IT), led […]
